Imprinting and the Epigenetics of the Brain and Sleep
Imprinting and the Epigenetics of the Brain and Sleep
Two reviews make genomic imprinting a key factor in brain development and sleep.
According to the 2016 Annual Review of Neuroscience, “the
brain has emerged as a main target of genomic imprinting, generating
great interest in how this epigenetic regulation provides stable
transcriptional control of neural development and behavior.” The authors add, “the importance of imprinted genes
in brain function is evidenced by the devastating neurological and
behavioral conditions” resulting from mutations in the genes concerned.
 As this review notes, imprinting—the expression of a gene from just
one parental copy rather than the other—is the key to two, otherwise
seemingly unconnected syndromes: Prader-Willi and Angelman. Prader-Willi
syndrome results from loss of a run of paternally expressed genes on
chromosome 15. Angelman syndrome is linked to the opposite pattern of
imprinting, and to diametrically opposite symptoms (left).
As this review notes, imprinting—the expression of a gene from just
one parental copy rather than the other—is the key to two, otherwise
seemingly unconnected syndromes: Prader-Willi and Angelman. Prader-Willi
syndrome results from loss of a run of paternally expressed genes on
chromosome 15. Angelman syndrome is linked to the opposite pattern of
imprinting, and to diametrically opposite symptoms (left).
Among these diametric differences is the finding that whereas Angelman children are wakeful and hyperactive, Prader-Willi ones are sleepy and lethargic (something which fits the conflict model of imprinting if you see the former as every mother’s worst fear and the latter as more preferable to her). Furthermore, as another recent study points out,
This author concludes, “Taken together, these results indicate that both maternal and paternal imprinted genes significantly control REM sleep, which may occur through the control of circadian variations of thermoregulation.” Indeed, there is also evidence that imprinting may affect the chief psychological concomitant of REM, or rapid-eye-movement sleep, dreaming: When brain centers are active in dreams where paternal genes are preferentially expressed, such as the hypothalamus and amygdalas, aggressive impulses on the part of the dreamer emerge. However, when predominantly maternal brain centers are activated in dreaming such as the forebrain and neo-cortex, aggressive impulses are inhibited and co-operative and pro-social ones expressed.*
As I also pointed out in a recent post, Igf2 is the classic imprinted gene, coding for insulin-like growth factor 2 (IGF2) and iconically represents the father’s genetic self-interest in his offspring’s consumption of maternal resources during gestation and growth by being paternally expressed. And just as graphically, maternally-expressed Igf2r in mice contradicts it, as diagrammed in another post. Indeed, as the Annual Review article notes, regulation of adult weight by imprinted genes generally involves maternally expressed genes contributing to reduced weight as a function of metabolic rate and food intake, whereas paternally expressed genes contribute to increased weight. Nevertheless, IGF2 is expressed from both parents’ copies in the brain’s choroid plexus and vascular compartments, and is preferentially maternally expressed in other regions of the brain whose superior size may be critical to their interactions with the largely paternally prescribed limbic brain, as I explained in the same post.
Nor is the role of Igf2 limited to control of growth. After a memory is acquired, it remains labile for several hours during its consolidation. In the rat hippocampus, IGF2 is increased in the hours after training, which is necessary for memory consolidation. Moreover, administration of this growth hormone after training enhances memory consolidation. Memory retrieval within a few days of learning returns it to a labile state, allowing its consolidation, which is also enhanced by IGF2 administration. IGF2 also promotes adult neurogenesis and is down-regulated in animals exhibiting depressed behavior after chronic stress. Significantly, over-expression of IGF2 in the dentate gyrus reverses these behaviors.
As the authors note,
The mid-brain dopaminergic system plays a critical role in the control and modulation of emotional, motivational, and cognitive behaviors as well as voluntary movements. The development of this system is targeted particularly by imprinted genes. In the developing brain, neuronal migration to appropriate sites is essential for the establishment of proper identity and functional connectivity. As the authors explain in detail,
this is mediated by cellular processes that are highly influenced by genomic imprinting. For example, absence of the paternally expressed Peg3 gene results in increased apoptosis (programmed cell death) in the forebrain, striatal, amygdala, and hypothalamic regions. As the authors comment, “these abnormalities vary between males and females, suggesting that Peg3 function regulates the establishment of sexual dimorphisms.”
 As the figure above illustrates, studies of mouse mutants and human
disorders have uncovered the vast significance of genomic imprinting in
the postnatal brain, encompassing all essential adult neural functions
from key elements of synaptic transmission and plasticity to the control
of energy balance and metabolism, as well as emotional, social, and
cognitive behaviors. Synaptic transmission underlies the propagation of
information throughout the brain and directs proper wiring of neuronal
circuits. Multiple imprinted genes participate in baseline transmission
and activity-dependent modifications of neuronal excitability. Indeed,
genomic imprinting plays a key role in the regulation of growth and
energy balance during embryonic and placental development, as well as in
non-neuronal tissues. Ultimately, the brain controls energy balance and
metabolism of the entire organism, and this homeostatic function is
performed primarily by the hypothalamus, which senses internal energy
states and orchestrates visceral responses.
As the figure above illustrates, studies of mouse mutants and human
disorders have uncovered the vast significance of genomic imprinting in
the postnatal brain, encompassing all essential adult neural functions
from key elements of synaptic transmission and plasticity to the control
of energy balance and metabolism, as well as emotional, social, and
cognitive behaviors. Synaptic transmission underlies the propagation of
information throughout the brain and directs proper wiring of neuronal
circuits. Multiple imprinted genes participate in baseline transmission
and activity-dependent modifications of neuronal excitability. Indeed,
genomic imprinting plays a key role in the regulation of growth and
energy balance during embryonic and placental development, as well as in
non-neuronal tissues. Ultimately, the brain controls energy balance and
metabolism of the entire organism, and this homeostatic function is
performed primarily by the hypothalamus, which senses internal energy
states and orchestrates visceral responses.
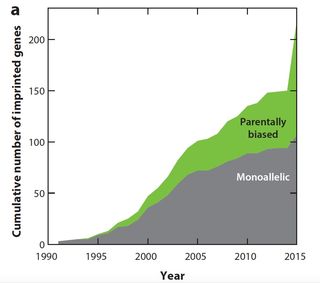
As the further figure left illustrates, the number of imprinted genes
discovered increases all the time. But as this review comments, genomic
imprinting is now seen as a continuum from classical one-parent control
at one extreme to weakly biased parental expression at the other. This
makes sense in view of the fact that genetic analyses of behavioral and
neurological defects highlight that normal brain development and
function require a fine regulation of gene dosages, so that even small
deviations may disturb the balance of the biological pathways they
control, leading to striking neural dysfunctions, as exemplified by
Angelman and Prader-Willi syndromes above.
Whatever else you might say, these reviews go a long way to establishing the fundamental claim that genomic imprinting is a key factor in sleep and brain development, both normal and pathological.

Source: C.Badcock
Among these diametric differences is the finding that whereas Angelman children are wakeful and hyperactive, Prader-Willi ones are sleepy and lethargic (something which fits the conflict model of imprinting if you see the former as every mother’s worst fear and the latter as more preferable to her). Furthermore, as another recent study points out,
Imprinted genes play a crucial role in the placenta and prenatal development and, after birth, have been demonstrated to control important metabolic and physiological functions (e.g., thermogenesis) as well as behavioural and cognitive processes. Imprinted genes have important roles during the perinatal period, which is a crucial time window in development, for the formation and integration of all biological systems, including the homeostatic control of sleep and the formation of the internal (circadian) clock.
This author concludes, “Taken together, these results indicate that both maternal and paternal imprinted genes significantly control REM sleep, which may occur through the control of circadian variations of thermoregulation.” Indeed, there is also evidence that imprinting may affect the chief psychological concomitant of REM, or rapid-eye-movement sleep, dreaming: When brain centers are active in dreams where paternal genes are preferentially expressed, such as the hypothalamus and amygdalas, aggressive impulses on the part of the dreamer emerge. However, when predominantly maternal brain centers are activated in dreaming such as the forebrain and neo-cortex, aggressive impulses are inhibited and co-operative and pro-social ones expressed.*
As I also pointed out in a recent post, Igf2 is the classic imprinted gene, coding for insulin-like growth factor 2 (IGF2) and iconically represents the father’s genetic self-interest in his offspring’s consumption of maternal resources during gestation and growth by being paternally expressed. And just as graphically, maternally-expressed Igf2r in mice contradicts it, as diagrammed in another post. Indeed, as the Annual Review article notes, regulation of adult weight by imprinted genes generally involves maternally expressed genes contributing to reduced weight as a function of metabolic rate and food intake, whereas paternally expressed genes contribute to increased weight. Nevertheless, IGF2 is expressed from both parents’ copies in the brain’s choroid plexus and vascular compartments, and is preferentially maternally expressed in other regions of the brain whose superior size may be critical to their interactions with the largely paternally prescribed limbic brain, as I explained in the same post.
Nor is the role of Igf2 limited to control of growth. After a memory is acquired, it remains labile for several hours during its consolidation. In the rat hippocampus, IGF2 is increased in the hours after training, which is necessary for memory consolidation. Moreover, administration of this growth hormone after training enhances memory consolidation. Memory retrieval within a few days of learning returns it to a labile state, allowing its consolidation, which is also enhanced by IGF2 administration. IGF2 also promotes adult neurogenesis and is down-regulated in animals exhibiting depressed behavior after chronic stress. Significantly, over-expression of IGF2 in the dentate gyrus reverses these behaviors.
As the authors note,
Neural stem cells (NSCs) of the ventricular zone proliferate asymmetrically to produce differentiated neurons or glia or to generate intermediate progenitor cells (IPCs), which further divide symmetrically to produce either IPCs or neurons (…). NSC fate decisions are highly regulated by imprinted genes through intricate control pathways.
The mid-brain dopaminergic system plays a critical role in the control and modulation of emotional, motivational, and cognitive behaviors as well as voluntary movements. The development of this system is targeted particularly by imprinted genes. In the developing brain, neuronal migration to appropriate sites is essential for the establishment of proper identity and functional connectivity. As the authors explain in detail,
this is mediated by cellular processes that are highly influenced by genomic imprinting. For example, absence of the paternally expressed Peg3 gene results in increased apoptosis (programmed cell death) in the forebrain, striatal, amygdala, and hypothalamic regions. As the authors comment, “these abnormalities vary between males and females, suggesting that Peg3 function regulates the establishment of sexual dimorphisms.”

Mouse phenotypes influenced by imprinted
genes. Genes associated with specific metabolic, social, emotional,
and cognitive phenotypes are listed. Genes preferentially expressed from
the maternal and paternal allele appear in red and blue,
respectively. Strongly biased
and mono-allelically expressed imprinted genes are in bold. Lines with
arrowheads and notched ends indicate stimulation and
inhibition, respectively, of the given phenotype, whereas lines
with square ends indicate functions that cannot be defined
as enhancement or reduction.
Source: Perez JD, et al. 2016, Annu. Rev. Neursci. 39:347-84
article continues after advertisement

Monoallelic and parentally biased genes in mouse.
(a) Cumulative increase in the number of imprinted genes reported in the
literature.
Whatever else you might say, these reviews go a long way to establishing the fundamental claim that genomic imprinting is a key factor in sleep and brain development, both normal and pathological.

